References
1. Bernard TN, et al. Recognizing specific characteristics of nonspecific low back pain. Clin Orthop Relat Res. 1987;217:266–80.
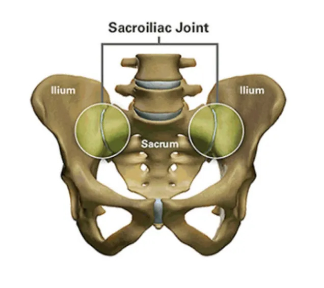
2. Schwarzer AC, et al. The Sacroiliac Joint in Chronic Low Back Pain. Spine. 1995;20:31–7.
3. Maigne JY, et al. Results of Sacroiliac Joint Double Block and Value of Sacroiliac Pain Provocation Tests in 54 Patients with Low Back Pain. Spine. 1996;21:1889–92.
4. Sembrano JN, et al. How Often is Low Back Pain Not Coming From The Back? Spine. 2009;34:E27–32.
5. DePalma MJ, et al. Etiology of Chronic Low Back Pain Patients Having Undergone Lumbar Fusion. Pain Med. 2011;12:732-9.
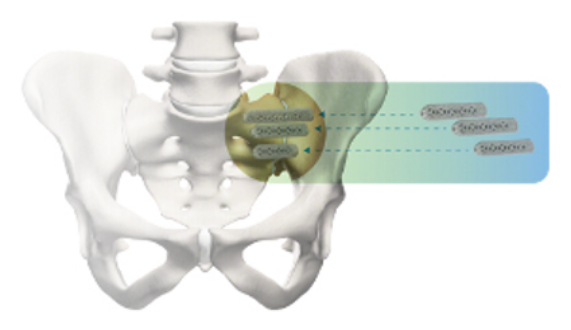
6. Polly DW, et al., and the INSITE Study Group. Two-Year Outcomes from a Randomized Controlled Trial of Minimally Invasive Sacroiliac Joint Fusion vs. Non-Surgical Management for Sacroiliac Joint Dysfunction. Int J Spine Surg. 2016;10:Article 28. DOI: 10.14444/3028
7. Dengler J, et al. Randomized Trial of Sacroiliac Joint Fusion vs. Conservative Management for Chronic Low Back Pain Attributed to the Sacroiliac Joint. J Bone Joint Surg Am. 2019;101(5):400-11. DOI: 10.2106/JBJS.18.00022.
8. Duhon B, Bitan F, Lockstadt H, Kovalsky D, Cher D, Hillen T, on behalf of the SIFI Study Group. Triangular Titanium Implants for Minimally Invasive Sacroiliac Joint Fusion: 2-Year Follow-Up from a Prospective Multicenter Trial. Int J Spine Surg. 2016;10:Article 13. DOI: 10.14444/3013
9. Dengler J, et al. on behalf of the INSITE, iMIA and SIFI study groups. Predictors of Outcome in Conservative and Minimally Invasive Surgical Management of Pain Originating from the Sacroiliac Joint – a Pooled Analysis. Spine. 2017;42(21):1664-73. [Epub 2017 Mar 27]. DOI: 10.1097/BRS.0000000000002169
10. Whang PG, et al. Long-Term Prospective Clinical and Radiographic Outcomes After Minimally Invasive Lateral Transiliac Sacroiliac Joint Fusion Using Triangular Titanium Implants. Med Devices (Auckl). 2019;12:411-422. DOI: 10.2147/MDER.S219862
11. Patel V, et al. Prospective Trial of Sacroiliac Joint Fusion Using 3D-Printed Triangular Titanium Implants: 24-Month Follow-Up. Med Devices (Auckl). 2021;14:211-216. DOI: 10.2147/MDER.S314828